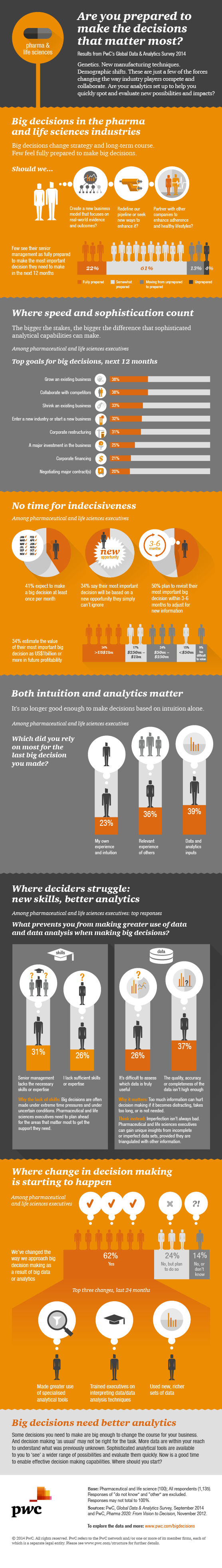
The pharmaceuticals industry — with its major investments in research, reliance on complex chemistry, and sophisticated understanding of human biology — is generally regarded as a technologically advanced sector. When it comes to manufacturing, however, pharma is stuck in the past. The current methods of making drugs, which are labor intensive and inefficient, are based on batch processes that have been in place in this sector since the mid-20th century. Worse still, the traditional manufacturing techniques make pharmaceuticals prone to contamination.
But that’s about to change, thanks to production innovations. A new approach called continuous manufacturing is on the verge of transforming the pharmaceutical value chain. It will affect every company in this industry, from giant multinationals to the third-party manufacturers that small startups hire to make their products. This shift in production capability will rapidly become “table stakes” for leading pharmaceutical firms. It has the potential to make drug manufacturing more efficient, less expensive, and more environmentally friendly. And it is not the only transformative innovation in this space. Digital fabrication — the so-called 3D printing of drugs — is also gaining traction as a viable technology for making small batches of medicines that have been too costly and impractical to produce.
Embracing such developments will be vital if the industry is to adapt to the pressures it faces today. The healthcare industry is in a state of flux. Global spending on healthcare has been soaring, and several countries have introduced initiatives intended to bring costs under control. The U.S. is still learning how to deal with the changes brought about by the Affordable Care Act. Around the world, reforms have unleashed numerous disruptive innovations, and growth in emerging markets is creating a large pool of prospective consumers.
In addition, pharma companies are struggling with challenges specific to their industry. Patents for many drugs — some of them blockbusters, which have created billions of dollars in revenue — have expired, and more will do so in the coming years. Health systems are no longer willing (or able) to pay what they used to for pharmaceuticals. Regulators and the public are asking pharma companies to produce and deliver more complex product portfolios at a lower cost, in more and more markets — while continuing to meet stringent quality requirements.
Amid such pressures, the industry has been slow to change its tried-and-true manufacturing methods. Why? Because chief operating officers have generally focused on making sure their high-margin products remained in stock and met quality requirements. So long as manufacturing costs were kept within industry norms, operating chiefs didn’t give much thought to them. Furthermore, changes in manufacturing processes often have regulatory ramifications. In most countries, all material changes to the way drugs are designed or produced need to be approved by the appropriate government agencies.
Continuous Manufacturing Takes Hold
Increasingly, however, the industry is being prodded to update its manufacturing approach and adopt new paradigms, and COOs are more focused on controlling costs. Drug companies are also under pressure to deliver a larger number of products to a wider range of markets. That’s where continuous manufacturing comes in.
In conventional pharma operations, drugs are produced in batches (rather than in assembly-line fashion, as cars are). Ingredients are mixed in large vats, in separate steps. Different parts of the process — the blending of powder ingredients, formation of pellets, compression into tablets, and coating — sometimes take place at different plants. Drugs are then packaged in a separate multistep process. The operation is time consuming, asset intensive, and expensive. The risk of contamination is always present because batches of partially finished medicines must be moved from place to place.
Continuous manufacturing technology breaks completely with this old methodology. It combines the segmented steps of batch manufacturing into one cohesive process, with more streamlined product flows and faster production times. Factories using this technology are designed for flexibility and for rapid, high-quality throughput, with more open floor plans and smaller footprints, and lower building and capital costs. The continuous model uses inline quality control to perpetually monitor what is being produced (instead of using traditional batch-based testing), which reduces the potential for contamination.
Continuous systems for pharma are still new, but they are showing very promising results. Many industry observers expect the first products made with this method to be introduced to the market in early 2016. Some of the established industry leaders are taking heed. GlaxoSmithKline plans to open a plant in Singapore in 2016 that will deploy a continuous manufacturing system, and leaders expect to cut both costs and carbon footprint by half, compared with those for a traditional manufacturing plant.
Novartis, a pioneer in such efforts, has partnered with the MIT Center for Continuous Manufacturing, and is investing US$65 million in a joint 10-year research project. The two parties have already concluded that continuous manufacturing will benefit patients, healthcare providers, and the pharmaceuticals industry. This project has demonstrated, for example, that continuous manufacturing can accelerate the introduction of new drugs through efficient production. It also tends to minimize waste, energy consumption, and raw material use, and to enhance companies’ flexibility in responding to market needs.
Continuous manufacturing has the capacity to allow pharma — which turns over inventory more slowly than most other major sectors — to catch up to companies in other fields, such as consumer products. With traditional batch manufacturing, production takes 200 to 300 days from the start of production to packaging and shipment to the pharmacy. Optimization can sometimes get this time down to 100 days. Continuous manufacturing, however, can produce a quantum leap, reducing throughput times to less than 10 days. Combined with the smaller plant sizes, this approach could reduce overall operating costs and capital expenditures by 25 to 60 percent, according to the researchers at the MIT-Novartis project (see exhibit).
Printing Medicine
Although continuous manufacturing is the wave of the near future, the advent of chemputing — what’s commonly called the 3D printing of drugs — is not far behind. 3D printing is already altering many processes and sectors, including the manufacture of clothing and toys and, in healthcare, the development of custom prostheses for amputees.
The technology also has the potential to revolutionize the pharma industry. Prototypes and projects have been in development for several years. In 2012, Craig Venter, the scientist best known for sequencing the human genome, unveiled a plan to develop 3D-printable vaccines. And University of Glasgow professor Lee Cronin has already started two companies that aim to develop and test processes for drug manufacture using 3D printing technology. Cronin’s device uses gel-based “inks” — including carbon, hydrogen, oxygen, vegetable oils, paraffin, and other ingredients — to create uniform molecules that can be combined in different formulations.
And in August 2015, the Food and Drug Administration approved the first ever 3D-printed prescription pill for consumer use, a treatment for epilepsy called Spritam, sold by Aprecia Pharmaceuticals. The new formulation dissolves significantly faster than a typical pill, which is a benefit to epilepsy patients, who may have trouble swallowing medication.
Production using these methods is well suited to drugs aimed at very small patient populations — those patients with “orphan diseases” or specific cancer mutations. The methods will thus advance the development of personalized medicine.
To stay current, pharmaceutical companies will need to embrace the new technologies. Rather than supplanting continuous manufacturing, 3D printing will likely work in tandem with it. This combination will give pharma companies great flexibility to produce different drugs in different ways, depending on their markets, their costs, and other specific requirements.
Davids and Goliaths
Big pharma companies are not the only ones that will feel the impact of these new technologies. Continuous manufacturing and chemputing are also game changers for relatively small pharmaceutical companies, including startups. Small pharma companies have typically found themselves challenged by manufacturing, with its high asset intensity and minimum efficient scale. But if the barriers to entry and the operating costs fall significantly, these companies will have a much easier time making their own drugs.
In the past, companies that couldn’t afford a global operations network had to outsource the production of their drugs to third-party contract manufacturers. The advent of cheaper, faster, safer drug manufacturing will ripple out to this group. To avoid obsolescence, they will need to embrace these innovations and enhance their own service offerings.
Supply chains will also evolve. With smaller factories and faster production cycles, pharma companies will be able to produce drugs much closer to where they’re needed. The industry can expect to see lower inventories, corresponding reductions in warehouse costs, and shorter transportation routes.
For the “Goliaths” of big pharma, this change comes not a moment too soon. Jonathan Rauch, a senior fellow at the Brookings Institution, noted in a
March 2015 paper that the industry’s endemic “cost-no-object, value-no-concern approach,” as he calls it, has made companies “seemingly impervious to disruptive innovations.” It has been impossible for competitors to break past the barriers to entry that exist, in part because of entrenched regulatory approaches and practices in the U.S., Europe, and elsewhere. However, he added, a growing culture of disruptive entrepreneurship is gaining a foothold, and manufacturing may be the most likely place to start.
The operations sector of the pharmaceuticals industry is now evidently willing and prepared to take the lead. And other observers agree that the short- and long-term winners will be not only the drug companies, both large and small, that smartly adapt, but also the patients whose medicines will become more effective and cheaper.
Although the first product of this system, as MIT-Novartis announced, could be out and approved soon, no one should expect that all drugs will soon be produced with the new technology. The coming decade will likely see a mix of traditional and continuous manufacturing techniques, as the older methods are phased out and newer technologies ramp up.
Pharmaceuticals manufacturing is like the airline industry at the beginning of the jet age in the mid-1950s. Companies may continue to function for the near term without upgrading their manufacturing technologies, just as many airlines kept flying propeller planes through the 1970s. But by 2025 (or sooner), the most successful pharma companies will be those that embraced today’s emerging manufacturing technologies.